Chapter 3, Minerals: The Building Blocks of Rocks - 09
Mineral Properties
To identify minerals without sophisticated apparatus, we rely on a set of characteristics that reflect the chemical constituents and atomic structure of the mineral. These are macroscopic observations:
These properties are established for most minerals. Thus if you have an unknown mineral, you perform a series of tests on that sample and compare them with the catalogued information on mineral properties.
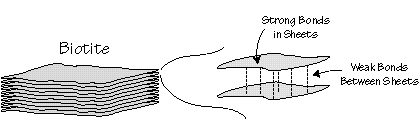
Often, atoms for strong bonds in chains or sheets, and these sheets are connected by weaker bonds. Materials constructed in this way cleave in preferential directions. That is, you can break them more easily in certain directions.
Cleavage is thus a direct consequence of the geometry and the type of atomic bonds within a mineral.
Cleavage is a consequence of fracture &endash; some minerals such as quartz can have beautiful planar surfaces, but they do not cleave well.
Color is not a unique identification tool of a mineral (but don't completely ignore it). Dark minerals are generally rich in iron and/or magnesium and are often called mafic. Lighter color minerals are usually feldspar and quartz and are referred to as felsic. Often the color of a mineral can be strongly affected by "impurities" and cannot distinguish mineral type.
The true color of the mineral shows up when a sample is powdered. The streak is a way of checking the color of a mineral's powder. You scratch the mineral on a porcelain plate (powdering part of it).
Luster is the shine of a mineral and reflects how light is reflected from the mineral. It can be metallic, pearly, glassy, or dull.
We can also classify minerals by how well light travels through them. Some mineral are opaque (no light gets through), others are translucent (some light transmission), transparent (see through).
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|