Chapter 2, Earth: Origin and Composition - 02
The Composition of the Universe
Atoms are the basic building blocks of matter (smaller divisions are possible, but for our purposes, the classic structure of atoms will do). Atoms are composites of three particle types:
The electron has a negative charge and orbits around the nucleus, which contains the positively charged protons and neutrons, particles that have no electric charge. Protons and neutrons are much larger and more massive than electrons (and are in fact made up of smaller particles called quarks).
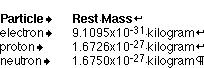
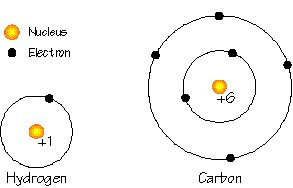
Atoms are mostly empty - a dense nucleus is surrounded by moving electrons. were the proton the size of a grain of sand, the electron shell would be the size of a football field.
Atoms can have different numbers of electrons, protons, and neutrons. We call atoms that have the same number of electrons and protons elements. The atomic number is defined as the number of protons in the atom.
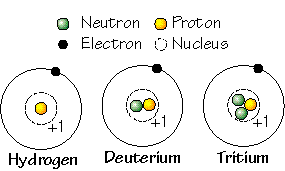
Atoms of a particular element can have a varying number of neutrons and we identify these variations of the elements isotopes. Some important elements in the study of geology are Carbon-12 and Carbon-13, and Oxygen-16 and Oxygen-18, and Uranium-235 and Uranium-238.
Molecules are structures that consist of bonded atoms, sometimes constructed of a single element, other times consisting of more than one element. Some commonly known molecules are water: H2O and sodium-chloride Na-Cl (table salt).