Chapter 2, Earth: Origin and Composition - 04
Atoms, Ions, and Elements
Rocks are made of minerals, minerals are made of atoms, and atoms are made of protons, neutrons and electrons.
In the small scale of atoms (about 10-10 meters), the elements are distinguished by the number of protons in the nucleus.
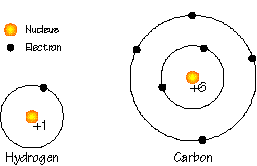
Each proton contributes one positive unit of electrical charge, each electron contributes one negative unit of electrical charge (neutrons have no charge).
If an atom has the same number of protons and electrons, it has no charge and is called neutral.
If an atom has an excess of electrons, it has a negative charge and is called an anion.
If an atom has an excess of protons, it has a negative charge and is called an cation.
Anions and cations are collectively referred to as ions.
When we talked about element formation in stars, we discussed changes in element nuclei. Chemistry is the study of changes in material behavior dependent on the electron configuration surrounding the nucleus.
The best way to envision electrons is to imagine them orbiting the nucleus in a set of pre-specified, "allowed" orbits.
The orbits are clustered into shells. The first shell has two orbits, the next eight, then two, then eight, then 18, etc.
We call the electrons in the outermost incomplete shell valence electrons. Atoms with valence electrons are more chemically reactive than those with no valence electrons (i.e. a complete shell).
Atoms really "hunger" for a configuration with a completely filled out electron shell. To fill outer shell, atoms will
The Periodic Table is a chart of elements arranged according to the electron configuration of the elements.
Elements in the same column of the periodic table need the same number of electrons to fill their outer electron shell and thus share similar chemical properties.
The electron shells fill from left to right, those on the left have only a few electrons in the outer shell. Those on the right have a full outer electron shell.
Elements with complete outer electron shells and don't react chemically. They are called the inert gases.
Elements on the far left react vigorously and are "eager" to give up their outermost electrons.
Elements in the inerior of the table often share their electrons with other atoms to achieve stability (low energy states).