Chapter 3, Minerals: The Building Blocks of Rocks - 10
Common Mineral Groups in Earth
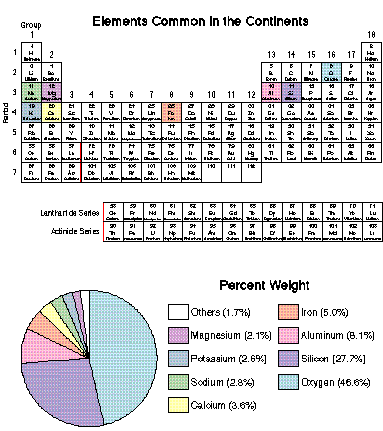
The continental crust is composed primarily of eight elements: O, Si, Al, Fe, Ca, Na, K, Mg.
The basic building block of the silicates is the silica-tetrahedron.
These tetrahedra can combine in chains, sheets, or complex three-dimensional structures to produce the silicate minerals that have a variety of properties.
We classify minerals based on the anions involved in the compounds.
|
Silicates |
Silica-Oxygen anions |
|
Oxides |
Metals (Fe, Al) with Oxygen e.g. Al2O3 &endash;> Ruby, Sapphire |
|
Carbonates |
CO32- anions (marine) Formed by marine life from elements in sea water. |
|
Sulfides |
Sulfur anions &endash; Form in oxygen-poor environments. e.g. PbS, galena. |
|
Sulfates |
Sulfur anions &endash; Form when oxygen is present. e.g. CaSO4´2H2O, gypsum. |
|
Halides |
Chlorine &endash; Salts, form by evaporation - NaCl, table salt. |
|
Native Elements |
Gold, Silver, Copper, etc. are rare, but precious. |
Earth's core is mainly iron and nickel with a small amount of a lighter element, perhaps oxygen or sulfur.
The Mantle is almost completely silicate, and is rich in magnesium. The minerals of olivine and pyroxene are most common, and an aluminum-bearing mineral. Three lines of evidence for upper mantle:
Earth has two types of crust - oceanic and continental.
Oceanic crust is composed of basalt, the result of partial melting of the mantle. It is rich in Mg, Fe, Ca, Al which are in the minerals olivine, pyroxene plagioclase feldspar.
Continental crust diverse, old, and composed of incompatible elements that do not fit well in the minerals stable in the mantle (K and Na). The elements are concentrated in low-density minerals such as quartz and feldspar.